zinc stearate reaction
The order of 25000 sqcm. For example the zinc stearate will melt during molding and be absorbed into the compound without leaving discoloration or defects on the surface of the final molded rubber part.

Measured Characteristics Of The Prepared Zinc Stearate Download Table
Eyes skin respiratory system NIOSH 2022.

. This chemical is an example of metal soaps and is insoluble in water and polar solvents such as alcohol. Excerpt from NIOSH Pocket Guide for Zinc stearate. In cosmetics zinc stearate is a lubricant and thickening agent used to improve texture.
As discovered in the early days of v. Adding zinc oxide four times and reacting at a temperature of 160DEG C under a pressure of 02Mpa for 50min. Method of producing zinc stearate involves reacting stearic acid and zinc hydroxide with heating and intense stirring followed by heat treatment filtration drying and packaging.
Inhalation ingestion skin andor eye contact. Dibasic zinc stearate Zinc distearate Zinc salt of stearic acid CAS No. Mainly used as lubricant and release agent for styrene resin phenolic resin and amino resin.
TABLE I FATTY ACIDS LENGTH LINEAR MELTPOINTC Lauric C12H24O2 saturated 44 Myristic C14H28O2 saturated 54. Reaction between ZnO and stearic acid forming zinc stearate and the molar masses of each chemical substance in the reaction. It is an activator for accelerated rubber sulfur vulcanization.
Water and zinc stearate were formed. By monitoring features in the infrared spectra that are characteristic of the global conformation of the hydrocarbon chain it is shown that the double. The Skin Deep scoring system was designed to help the public understand whether a product is safe to use or whether it contains ingredients of concern.
Every product and ingredient in Skin Deep gets a two-part score one for hazard and one for data availability. The invention discloses a kind of new process of producing Zinic stearas it is characterized in that citric acid acetate and water are mixed as catalyst for reaction. These applications exploit its non-stick properties.
For zinc and zinc oxide the first reaction zone began at about 160 C and extended to 280290 C. Consult your pharmacist or. The technology comprises the following steps.
The safest products score well by both measures with a low hazard rating and. It is a white powder and is insoluble in water. Zinc stearate is an organic substance with a chemical formula of C36H70O4Zn.
Zinc stearate was synthesized by precipitation method through two steps. It is widely used as a release agent for the production of many kinds of objects. ZnO reacts with the StH to form zinc stearate which is soluble in the rubber and facilitates the cross-linking reaction.
From 280 to 375 C zinc stearate was present in the reaction products of the acid with both zinc metal and zinc oxide. The aqueous emulsion of zinc stearate is called zinc. 1 stearic acid catalyzer and zinc oxide poured into successively mix.
Three reaction parameters including temperature time and amount of HCl per zinc stearate were varied to optimize the acid-catalyzed esterification reaction of zinc stearate while addition of toluene was regarded as an essential parameter. Zinc stearate is produced from various reactions including the reaction of zinc oxide and stearic acid which is shown in the following. Product to use on the surface of the uncured rubber.
Dibasic zinc stearate Hydense Hytech Mathe Metallac Metasap 576 Octadecanoic acid zinc salt Petrac ZN-41 Stavinor ZN-E Stearates ACGIH Stearic acid zinc salt Synpro stearate Talculin Z Zinc distearate Zinc octadecanoate Zinc stearate ACGIHOSHA Acute Toxicity Data and References. A catalyst paste containing calcium hydroxide zinc oxide and zinc stearate in ethylene toluene sulfonamide reacts with a base paste containing calcium tungstate calcium phosphate and zinc oxide in glycol salicylate to form an amorphous calcium disalicylate. At 600 C no acid soap was detected.
Conveying liquid stearic acid by a pump allowing the liquid stearic acid to go through a flow meter for metering and then enter a zinc stearate reaction vessel stirring and heating. When reacting stearic acid and zinc hydroxide hydrochloric acid is. In Canada - Call your doctor for medical advice about side effects.
It can also act as a lubricant mold release agent and dusting agent for rubber preventing the rubber from sticking to the mold as well as to. DOT ID Guide. Zinc stearate Zinc distearate is a fine white soft and muctuous odourless bulky powder molecular weight 632.
Adding molten zinc stearate into a tablet. You may report side effects to Health Canada at 1-866-234-2345. Zinc stearate has different ratios of palmitic and stearic acids.
Irritation eyes skin upper respiratory system. NMR and GC-MS measurements on methyl-esterified products from zinc stearate were used to evaluate each. The influence of a double bond in the middle of an otherwise flexible hydrocarbon chain on the melting of such assemblies has been investigated by comparing the melting behavior of zinc stearate and zinc oleate.
Consult your pharmacist or physician. Rubber polyurethane polyester processing system powder metallurgy. Neutralization of stearic acid by sodium hydroxide then double decomposition using zinc sulphate to precipitate zinc stearate.
Its outstanding characteristic is the extremely small particle size of the top quality material which can be less than one micron in diameter giving it a high specific surface eg. At the same time it also has the function of vulcanization activator and softener in rubber. As an anti-adhesive and anti-tacking agent Zinc Stearate reduces adhesion between rubber surfaces and in rubber products.
With the high-speed mixer is reactor reaction raw materials proportioning stearic acid.

Reaction Between Zno And Stearic Acid Forming Zinc Stearate And The Download Scientific Diagram
Zinc Stearate C36h70o4zn Pubchem

Effect Of Zinc Stearate On The Properties Of A Sulfonated Polystyrene Ionomer Journal Of Rheology Vol 62 No 4

The Chemistry Of Limescale Teaching Chemistry Chemistry Education Chemistry

Failure Mechanism Of Zinc Adipate As A B Nucleating Agent For Polypropylene In The Presence Of Calcium Stearate Sciencedirect

Zinc Stearate Technical Grade 557 05 1

Hascovir Control Max 0 4g X 30 Tablets Uk Headache And Dizziness Cold Sore Atopic Skin

Pdf Zinc Stearate Production By Precipitation And Fusion Processes Semantic Scholar

Zinc Stearate Formation And Vulcanization Mechanisms For Step 1 Download Scientific Diagram

Polymers Free Full Text Design And Synthesis Of A New Mannitol Stearate Ester Based Aluminum Alkoxide As A Novel Tri Functional Additive For Poly Vinyl Chloride And Its Synergistic Effect With Zinc Stearate

Influence Of Two Different Alcohols In The Esterification Of Fatty Acids Over Layered Zinc Stearate Palmitate Sciencedirect

Novel Approach For Rapid Oil Water Separation Through Superhydrophobic Superoleophilic Zinc Stearate Coated Polyurethane Sponges Sciencedirect
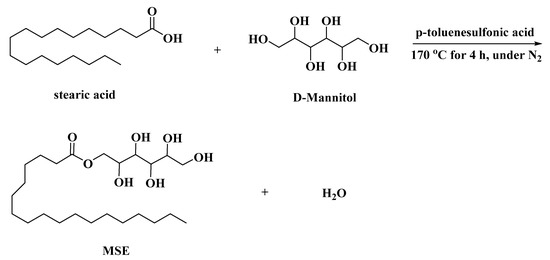
Polymers Free Full Text Design And Synthesis Of A New Mannitol Stearate Ester Based Aluminum Alkoxide As A Novel Tri Functional Additive For Poly Vinyl Chloride And Its Synergistic Effect With Zinc Stearate

Lpgat1 Controls The Stearate Palmitate Ratio Of Phosphatidylethanolamine And Phosphatidylcholine In Sn 1 Specific Remodeling Journal Of Biological Chemistry
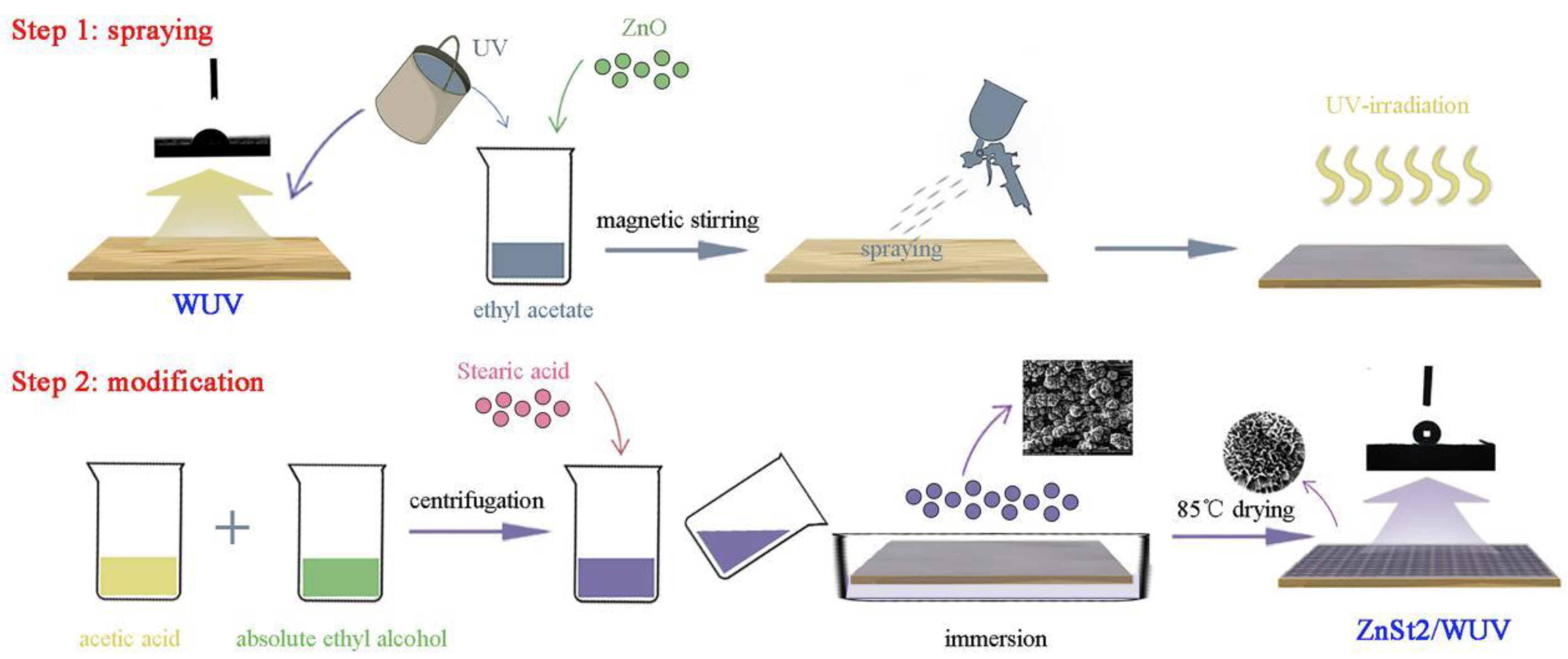
Polymers Free Full Text Preparation And Characterization Of Waterborne Uv Lacquer Product Modified By Zinc Oxide With Flower Shape Html

Comparison Of Commercial Zinc Stearate Reference Sample In Atr Mode And Download Scientific Diagram




Comments
Post a Comment